Healthy Volunteers Wanted to Test UK's First COVID-19 Vaccine
Imperial College invites local people to apply for trial at Hammersmith Hospital
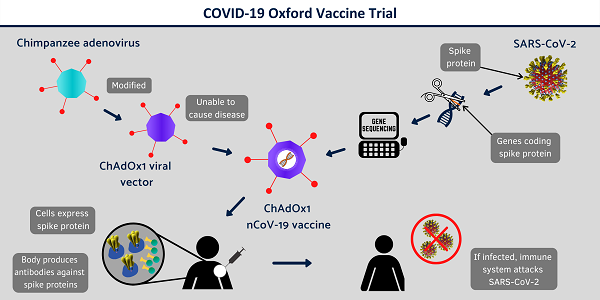
Oxford University and Imperial College are now inviting people living in South-West, West or North-West London to volunteer to take part in their COVID-19 vaccine trial.
If you are aged 18-55 and in good health, you could be eligible to participate.
If they pass screening, volunteers will be among the first humans to test the new vaccine, called ChAdOx1 nCoV-19.
The trial will involve up to 1112 volunteers. 1102 will be randomly sorted so that half will be given the COVID-19 vaccine and half will receive a control. They won’t know whether they received the COVID-19 vaccine or not until the trial is completed. The other ten volunteers will be given two doses of the COVID-19 vaccine.
The trial, which this week received an additional £20 million from the government, is taking place at multiple centres across the UK, including Southampton, Bristol and Oxford, where the first human trial is due to begin today, Thursday 23 April, and is expected to run for the next six months.
The trial will provide valuable information on the safety aspects of the vaccine, as well as its ability to generate an immune response against the virus.
To be eligible, volunteers must not have tested positive for COVID-19, be pregnant, intending to become pregnant, or breastfeeding during the study or have previously taken part in a trial with an adenoviral vaccine or received any other coronavirus vaccines.
The trial will last six months, with between four and 12 visits to the Imperial Clinical Research Facility at Hammersmith Hospital in White City, plus an optional visit after a year. Volunteers who complete the trial will receive between £190 and £625, depending in which group they are in.
Dr Katrina Pollock, Clinical Research Fellow in Vaccinology, who will be leading the work at Imperial’s Hammersmith campus, said: "This is the first-in human trial for this candidate vaccine and an important step towards developing a safe and effective vaccine against the novel coronavirus.
"Our clinical team is excited to begin trials this week as a key centre in this landmark study to combat COVID-19."
At the same time as conducting the first clinical trial, production of the vaccine is being scaled up ready for larger trials, and potentially, future deployment. By starting vaccine manufacturing scale-up immediately, the team can ensure that enough vaccine doses are available as soon as possible – especially for NHS workers, the elderly, and those with underlying health conditions – if the trials prove that the vaccine is safe and effective.
Dr Sandy Douglas, who is leading on the vaccine manufacturing scale-up project, said: "The scale of this epidemic poses a huge challenge for vaccine manufacturing. We need to follow rigorous safety standards and that takes time. By starting work on large-scale manufacturing immediately, we hope to accelerate the availability of high quality, safe vaccine."
Professor Teresa Lambe leading the early stages of our vaccine development said: "The commitment, compassion and helpfulness felt throughout the whole effort from everyone we have been working with has been amazing. We deeply appreciate the support of all our collaborators, funders and the teams around us in getting to this stage with the speed we have."
Interested individuals can volunteer to participate on the COVID-19 vaccine website and find further information on what the trials involve at Information for Participants.

Volunteers are also being invited to apply for a separate vaccine trial which is being let by Professor Robin Shattock at Imperial College London. It will be tested in human trials beginning in June, also at the NIHR Imperial Clinical Research Facility in Du Cane Road in White City.
Professor Shattock’s team has been testing an RNA vaccine candidate in animals since early February. When injected, the self-amplifying RNA vaccine will deliver genetic instructions to muscle cells to make the ‘spike’ protein on the surface of the coronavirus. This should provoke an immune response and create immunity to COVID-19.
This week the project was given £22.5 million to fast-track development of the vaccine and the team will soon begin screening participants for the trial.
Professor Robin Shattock said: "This investment will help us accelerate our clinical programme moving from starting human safety trials in June through to testing whether the vaccine can prevent infection in the wider community. We are working as fast as we can to determine the vaccine’s efficacy and to get to a position where millions or billions of the vaccine can be manufactured rapidly.
"We are tremendously grateful to the UK government and the British people for their investment in this vital work. We are also thankful for the philanthropic support to date that has helped us navigate funding blockages and which we hope will allow us to make this vaccine available worldwide, including to people in low- and middle-income countries."
Members of the public can register their interest by signing up via the NIHR Imperial CRF Healthy Volunteers Database.
April 23, 2020